Understanding the states of matter is essential for understanding the behaviour and characteristics of substances in the fields of physics and chemistry. Solids, liquids, and gases are the three main states in which matter may be found. We shall set out on an exploration of these states of matter in this blog post, illuminating their traits, transformations, and uses.

This article will provide you a thorough grasp of the many states of matter whether you’re a student at Cambridge Global Classes (CGC), one of the top coaching centres in Hyderabad run by Stanford and IIT graduates, or a student registered in the CBSE or IGCSE educational boards.
Solids: Stability and Structure
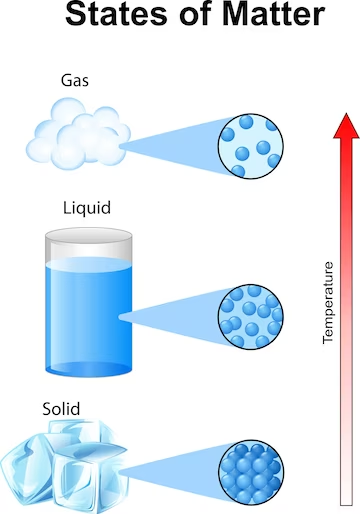
Solids have fixed shapes and volumes that define them. In solids, the particles are closely clustered and display strong intermolecular forces that keep them in place. Solids gain their stiffness and stability from this configuration. Solids include things like metals, wood, rocks, and ice. Solids are appropriate for use in manufacturing, building, and other industrial applications due to their unique characteristics.
Liquids: Flow and Fluidity
Liquids, unlike solids, do not have a definite shape but possess a definite volume. The particles in liquids are loosely packed, allowing them to move and flow past one another. This mobility gives liquids their ability to take the shape of their containers.

Liquids have weaker intermolecular forces compared to solids, allowing them to flow and exhibit fluidity. Common examples of liquids include water, oil, and milk. Liquids find extensive use in our daily lives, from hydration and cooking to transportation and lubrication.
Gases: Expansion and Dispersal
Gases are the most unconfined state of matter, lacking both definite shape and volume. Gas particles are widely spaced and move rapidly in all directions. They possess high kinetic energy, allowing them to overcome intermolecular forces and expand to fill their containers.

Gases are highly compressible and can be easily compressed or expanded. Examples of gases include air, oxygen, and carbon dioxide. Gases play a crucial role in industries such as power generation, chemical manufacturing, and refrigeration.
Phase Transitions: Changing States
Matter can undergo phase transitions, transforming from one state to another under specific conditions. These transitions include melting, freezing, evaporation, condensation, sublimation, and deposition.
For example, when heat is applied to ice (solid), it melts and becomes water (liquid). Further heating causes water to evaporate and become steam (gas).
Similarly, when steam is cooled, it condenses into liquid water, and upon further cooling, it freezes into ice. These phase transitions are governed by energy exchange and changes in intermolecular forces.
Applications and Significance
In many scientific and technical domains, an understanding of the states of matter is essential. Understanding thermodynamics, fluid mechanics, and material qualities are made easier by the study of states of matter in physics.
It supports the investigation of chemical kinetics, substance behaviour, and chemical processes in chemistry. Industries rely on state-of-matter understanding to create effective processes, manage reactions, and create new materials.
The study of matter’s three basic states—solids, liquids, and gases—reveals the various characteristics and behaviours that various substances display.
These states of matter influence our daily lives in a variety of ways, including the stability and structure of solids, the flow and fluidity of liquids, and the expansion and dispersion of gases.
