The elements, which serve as the basic building blocks of matter, are represented by the periodic table, a legendary masterpiece of scientific arrangement. It offers a well-organised framework that enables scientists to comprehend and categorise the wide variety of components present in the cosmos.
We shall explore the significance of the periodic table’s organisation and go on an expedition through it in this blog article. Your comprehension of the periodic table and its significance in the study of chemistry will be improved by this essay.
Organization and Structure
According to their atomic number, elements were arranged in the periodic table, which represents the number of protons in an atom’s nucleus. Elements are arranged in rows called periods and columns called groups or families. The table’s structure allows for the identification of patterns, trends, and relationships among elements, providing a comprehensive overview of their properties and behaviour.

Elements and their Properties
The periodic table consists of over 100 elements, each with unique characteristics and properties. The table is divided into several blocks: s-block, p-block, d-block, and f-block. Elements in the s-block and p-block are called representative elements, while those in the d-block and f-block are transition elements and inner transition elements, respectively.
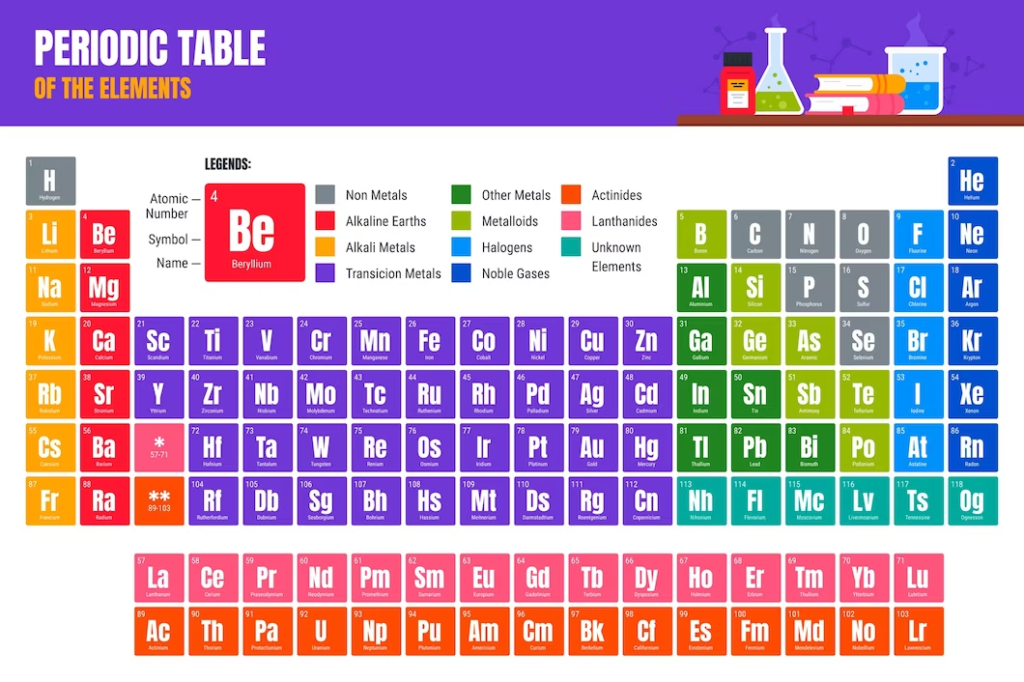
Elements within a group share similar chemical properties. For example, Group 1 elements, known as alkali metals, are highly reactive and have a single valence electron. Group 18 elements, known as noble gases, are chemically inert due to their stable electron configurations. Understanding the periodic table enables chemists to predict an element’s properties based on its position within the table.
Periodic Trends
The periodic table reveals various trends and patterns that govern the behaviour of elements. Some important trends include atomic radius, ionisation energy, electronegativity, and metallic character. These trends provide valuable insights into the reactivity, bonding, and physical properties of elements.

Application and Significance
The periodic table is the foundation of modern chemistry and finds extensive applications in scientific research, industry, and technology. It enables scientists to identify and understand the properties of elements, predict chemical reactions, and develop new materials.
In industries including pharmaceuticals, materials science, environmental research, and energy generation, the table is crucial.
New elements have been discovered in large part because of the periodic table. Scientists have successfully synthesised elements that were previously unknown or unstable by spotting gaps and trends in the table. This continual effort to enlarge and improve the periodic table advances our knowledge of the basic makeup of stuff.
The periodic table is an effective tool for comprehending and categorising the elements, revealing the mysteries of their characteristics and behaviour.
We learn more about the intricate links and patterns that the elements display by investigating the periodic table. This arrangement of elements provides a road map for scientific inquiry and technological developments, from atomic structure to chemical reactivity.
Let’s continue to use the periodic table as a tool to explore the mysteries of the elements and open new avenues for research and advancement.
